Medical Devices have always been an object of concern and regulation by the Indian Government. With advancing medical technology and the exponentially rising need of medical devices in the country, new reforms and rules have come into effect. These devices have been categorized into A, B, C and D classes and are governed by respective central and state regulatory authorities. Regulatory bodies classify medical devices based upon the associated risk and have defined relatively easy procedures for license applications. Similarly, rules and guidelines have been promulgated by the government for the import of medical devices. Whereby, all one needs is to have an authorized Indian agent to apply for the license.
With the reformative intervention and efforts of the government such as the SUGAM portal, obtaining medical device licenses is now an expedite procedure. We at CliniExperts strive to provide medical device regulatory services to our clients assistance with application and licensing procedures related to Medical Devices.
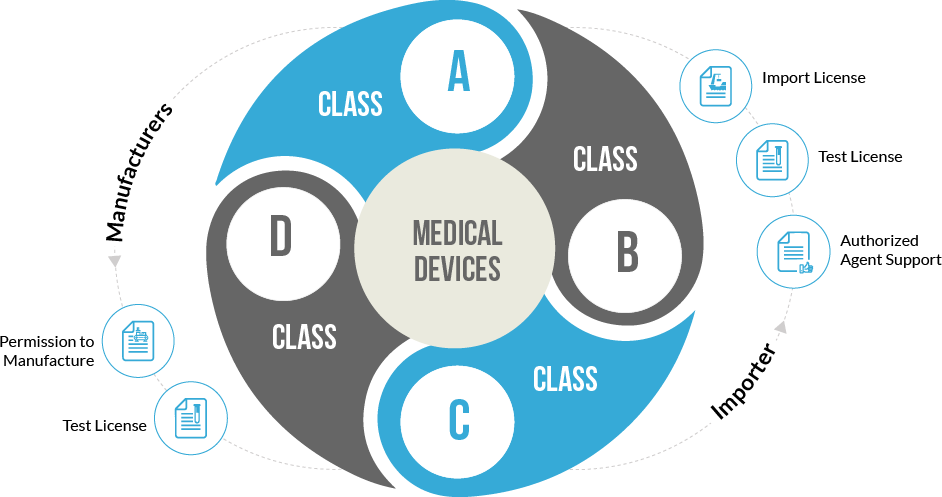
As per government rules, a medical device which itself or its predicate hasn’t yet been included in the medical devices list of CDSCO (Central Drugs Standard Control Organization) is considered a new medical device. Moreover, already registered devices vying to apply for new claims with respect to different implementation factors will also be treated as new devices.
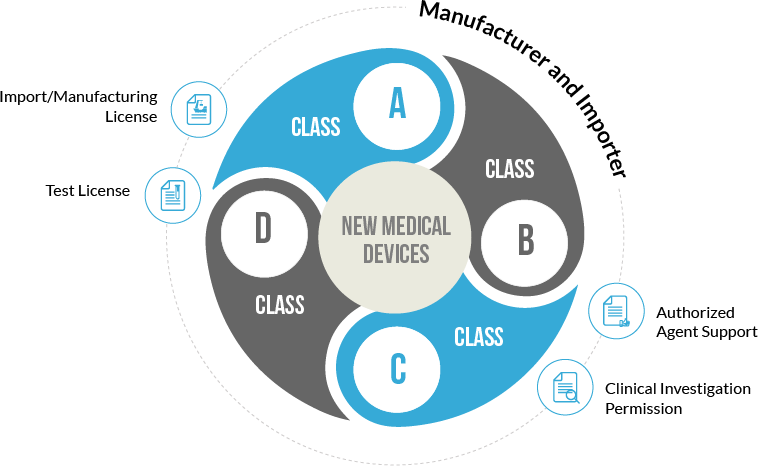
Following the latest reforms, medical devices have been classified into 4 major categories based upon their usage risk and further classified into surgical and non-surgical equipment.
Medical devices have been classified into A, B, C and D categories where the risk factor involved increases from A to D. Low-risk devices include equipment like thermometers whereas high-risk devices include pacemakers, heart valves and others. The devices are further classifed as surgical or non-surgical devices based upon their invasiveness. License for class A devices is easy to obtain as compared to class D devices.
The Central Drugs Standard Control Organization has formulated a set of forms to receive application for medical device licenses. These forms differ based upon the intended purpose of application. Different forms have been classified for importers and manufacturers, further diversifying them upon the basis of risk factor associated with different medical devices. Applying for individual medical devices is easy whereas application for multiple devices for import or manufacturing requires a great deal of attention.
| Applicant | Risk/Class | Type of Licence | Forms |
|---|---|---|---|
| Importer | A, B, C, D | Importer License | Application: MD-14 Permission: MD- 15 |
| Manufacturer | A, B | Manufacturing License | Application: MD-3 Permission: MD- 5 |
| Loan License | Application: MD-4 Permission: MD- 6 |
||
| C, D | Manufacturing License | Application: MD-7 Permission: MD- 9 |
|
| Loan License | Application: MD-8 Permission: MD- 10 |
| Applicant | Risk/Class | Type of Licence | Forms |
|---|---|---|---|
| Importer | A, B, C & D | Clinical Investigation Permission |
Application: MD-22 Permission: MD- 23 |
| A, B, C & D | Import License | Application: MD-26 Permission: MD- 27 |
|
| A, B, C & D | Test License | Application: MD-16 Permission: MD- 17 |
|
| Manufacturer | A, B, C & D | Clinical Investigation Permission |
Application: MD-22 Permission: MD- 23 |
| A, B, C & D | Manufacturing License | Application: MD-26 Permission: MD- 27 |
|
| A, B, C & D | Test License | Application: MD-16 Permission: MD- 17 |
With the recognition of India as a prominent global market and fast growing economy, international companies are now vying to grab a share of the Indian medical device market. In this wake, the Indian government has revised its respective laws and regulations to create a more friendly environment for medical imports. Authorities have promulgated rules to include and classify new medical devices in-line with the growing global trends and technology.
The process for granting licenses has been simplified and paced to allow quick launch of products in the market. The documentation and procedures have been aligned with global formats and conventions to unify and simplify procedures.

Classification
of Medical Devices


Aplication Filing
(Form MD- 14)

Import Licence
(Form MD- 15)
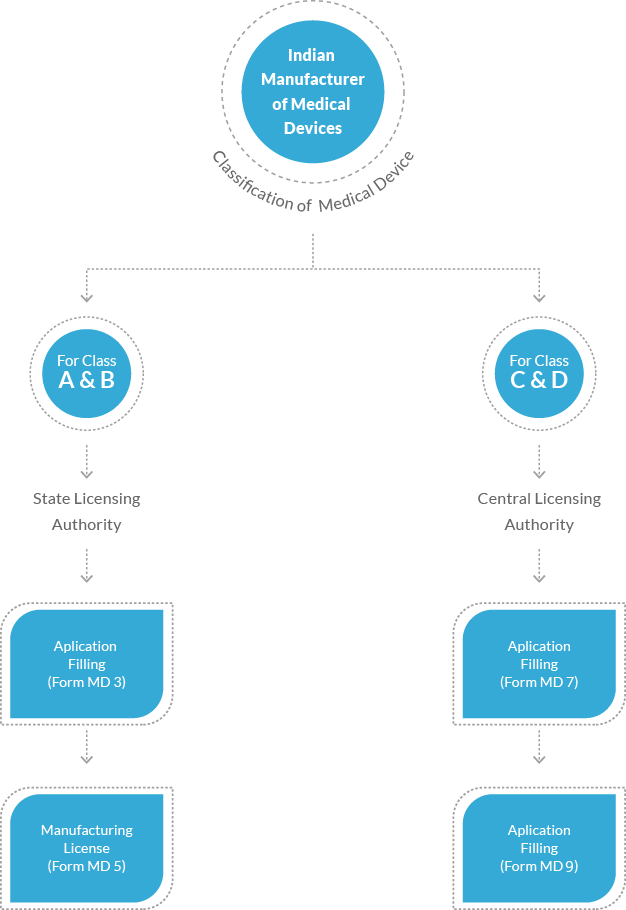
New rules and regulations have been promulgated by the authorities for manufacturing medical devices in India. The licensing authorities for filing applications vary based upon the classification of medical devices. Manufacturers aiming to manufacture Class A and Class B medical devices will be entertained and granted licenses by the State Licensing Authority. Whereas applications of manufacturers vying for Class C and Class D medical devices will be reviewed and granted permission by the Central Licensing Authority. CDSCO has also predefined appropriate application fees for different medical devices.
A new medical device whose similar or predicate is not available in India, or any other existing medical device with a change in design or intended use, needs to demonstrate their safety before applying for the license. In this accord, a clinical investigation has to be conducted on human participants to evaluate the effectiveness and safety of the medical device. Once the clinical investigation has been completed and the device is regarded safe, an application for the import or manufacture of this new investigational medical device can be filed with the Central Licensing Authority (CLA).
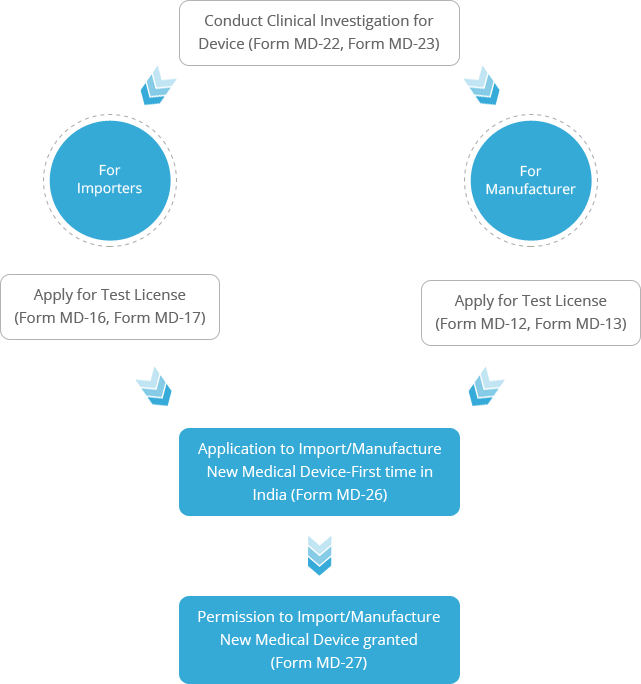
Clinical Investigation is required to be conducted in human participants in India for the
following conditions:
medical devices do not require a clinical investigation hence the application for their import or manufacturing can be filed directly.
medical devices which have not been granted a free sale certificate by any of the GHTF countries (U.S, Canada, EU, Japan and Australia) inevitably require a clinical investigation to be conducted.

Regulation/Guidelines
With the spurt in demand for infrared thermometers in India due to Coronavirus pandemic, the Government of India has eased down procedures at the custom level for easier import of the non-contact infrared thermometers by issuing no-objection certificate (NOC) for release of these medical devices.
Read MoreRegulation/Guidelines
The new ‘Medical Devices Rules, 2017’ have already been published and will commence w.e.f January 1st, 2018. A guidance document has been issued by the Ministry of Health & Family Welfare (MoH&FW) for the said rules under the ‘Rule 6’ after consulting medical devices & in-vitro diagnostic devices (IVD) industry […]
Read MoreNews
The Drugs and Cosmetics Act, 1940, has been governing drugs and medical devices since 1940 and has received multiple iterations each year. Owing to the significant changes that have occurred in the healthcare and pharmaceutical industry and constant requests from leaders in these industries, an overhaul was long overdue. In […]
Read More
Regulation/Guidelines
World Health Organization (WHO) has declared Corona virus Disease (COVID -19) as a global pandemic. A recent coronavirus outbreak is a public health emergency of international concern due to its rapid transmission and spread. So far, till March 1, 2020 there have been 191,127 confirmed cases, and among them, 7807 […]
Read More
The Medical Devices Rules (MDR), 2017 came into effect from 1st January, 2018. On February 11, 2020, two major notifications related to the Medical Devices Rules, 2017 were published by the Government of India. The notifications included: A new definition of medical devices. The Medical Devices (Amendment) Rules, 2020 As […]
Read MoreThe Indian healthcare industry is one of the world’s largest healthcare setup. Being the 2nd largest population in the world, the healthcare of this burgeoning people is funded by either healthcare insurance, government, employer but largely by the patient themselves. With increasing interest by the Indian Government to organize and […]
Read MoreNowadays, therapeutic treatment based on medical devices is providing technologically advanced solutions for the management, diagnosis, treatment,mitigation or prevention of several diseases. Thus, the demand continues to grow in the market at a tremendous rate leading to a renewed interest in the scientific development and research in the field of […]
Read More